A clinical research associate is a healthcare professional who performs many activities related to medical research and clinical trials. Participants are provided with information about the clinical trial.

A clinical research associate works both at clinical sites and sponsor locations.
Roles and responsibilities of cra in clinical trials. The people who play these parts are major components of what is needed to carry a clinical trial from start to finish. Difference between clinical research coordinator and clinical research associate: Clinical research associate also known as monitor is employed by either a pharmaceutical company or a contract research organization (cro) which works on behalf of pharmaceutical companies.
The content of the informed consent is explained. Protect the integrity and confidentiality of records. The role of a study coordinator in clinical trials is very important.
Role of crc after the clinical trial: Creating and writing trial protocols, and presenting these to the steering committee. The primary responsibility of a clinical research associate (cra) is to monitor clinical trials that are ongoing at clinical trial sites.
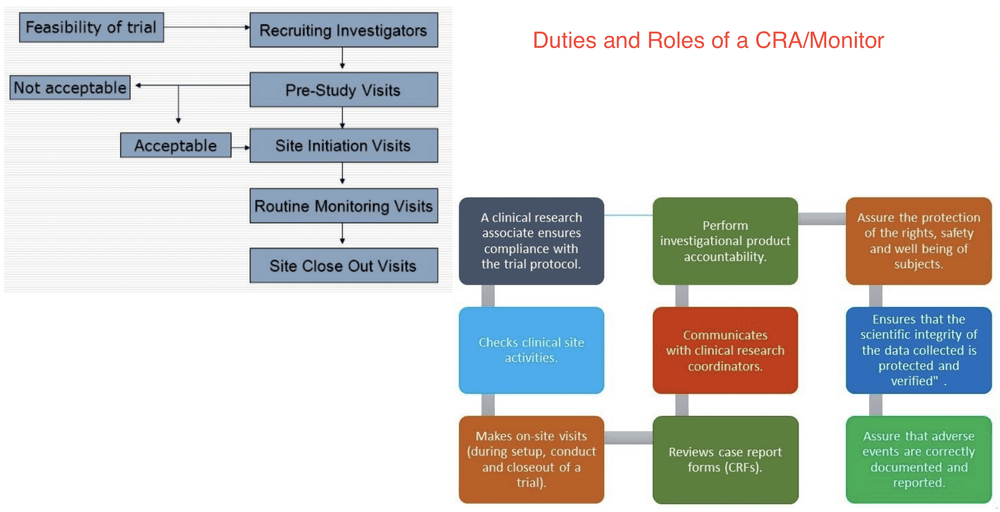
One of the positions with which people may be less familiar is that of the clinical research associate (cra). Clinical research associates should assist in organizing and monitoring the different stages of clinical trials. Cra / monitor who is cra?
Visit & work with sites on behalf of sponsor performs variety of clinical operations & monitoring activities often called as ‘clinical monitor’ or ‘study monitor’ 2. Their responsibilities revolve around preparing and processing necessary documentation. Training site staff on therapeutic areas, protocol.
A clinical research associate is a healthcare professional who performs many activities related to medical research and clinical trials. Participants are provided with information about the clinical trial. A clinical trial associate is in charge of coordinating and executing clinical trial operations, ensuring to meet all goals within budgets and deadlines.
A clinical trial manager reports to a clinical project. Roles and responsibilitie 01 investigator 03 sponsor 02 coordinator 04 monitor 05 contract research organization. The following are other duties and responsibilities a clinical research associate should be able to execute:
Roles and responsibilities in clinical trials of investigator, study coordinator, sponsor. Originally the functions and responsibilities of the msl role were more focused on the late phases of the clinical research (phase iii and beyond). There’s also trial coordination, regulatory management, data processing, medical research, and so much more.
Identifying, evaluating, and establishing trial sites, and closing sites down on completion of the trial. Clinical trials leverage the expertise of people in a wide variety of roles — researchers, project managers, clinical data managers, etc. The cra (s) in accordance with the sponsor’s requirements should ensure that the.
Overview responsibilities job descriptions resume examples skills & personality traits. When relevant and necessary to the trial and the trial site: Clinical trial managers execute and supervise clinical trials, and oversee and guide clinical research associates (cra) and clinical data managers (cdm).
Often a busy, working physician running a trial on the side) is chosen to conduct a trial at their site, clinical research coordinators often take over part of the essential responsibilities of pi.this includes making sure the trial is 1) conducted and 2) in. Before a clinical trial is completed at the sites, the study crc has to. The duties and responsibilities of.
Learn about the key requirements, duties, responsibilities, and skills that should be in a clinical trial manager job description. Every clinical trial site may have one or more study coordinators depending on the workload at the. When a pi (principal investigator, i.e.
Acting as the main line of communication between the sponsor and the. A clinical research associate works both at clinical sites and sponsor locations. Cras play a critical, if somewhat unheralded, role in the success of a trial.
However, the survey data also reflects that, step by step, the involvement of the msl regarding the sponsored clinical trials and iits has been changing, becoming more relevant and contributory to. The investigator must also be aware of any local rules or Cras are often responsible for multiple trials at one time, meaning significant amounts of travel between these sites.
Reporting of adverse events or drug reactions. Supervise study sites and activities to ensure adherence to appropriate industry protocols and terms of the study. Conducting a clinical trial requires much more than just a doctor and a patient.
Acts as main line of communication between sponsor and the investigator. Responsibilities of investigators can be found in the following sections of the regulations: The following is a selection of questions and answers excerpted from the centerwatch.
In addition, crcs have a critical role in protecting human subjects in clinical trials. Their job description entails monitoring clinical trials, working directly with the sponsor company as an independent freelancer or for a contract research organization. Trial is conducted and documented properly by carrying out the following activities.